
In the first white paper in our series “Growing Up Biotech”, we discuss the importance of communicating a compelling product story early in a biotech’s development.

PAGs play an increasingly important role in the drug development and regulatory process. Companies that truly want to be patient-centered should engage these groups early, often, and enthusiastically.
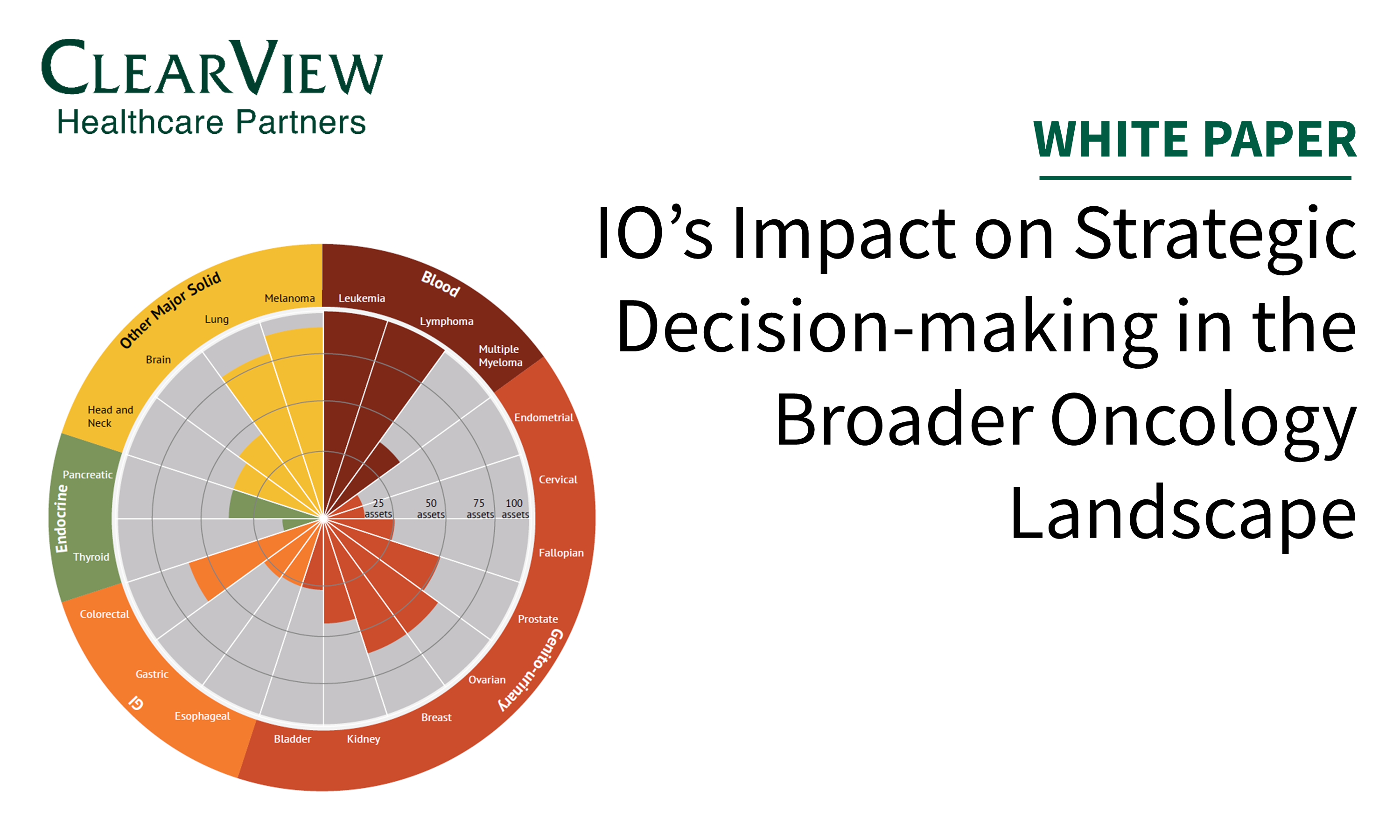
In this article published in In Vivo, we review key strategic considerations across clinical development, market access, and post-launch commercialization important for oncology drug development.

What are the clear areas of opportunity and the unique challenges associated with orphan disease drug development to help companies be best prepared to succeed in this exciting, but nuanced, space?

Informed drug development in an era of “precision medicine” requires a clear understanding of the risks and benefits that accompany the pursuit of companion diagnostics, a difficult objective given the complexity and rapid evolution of the field.

What are the key advantages and disadvantages for a biopharmaceutical company to consider prior to pursuing the breakthrough therapy pathway?